Interaction of the hub protein DYNLL with LC8 peptide
LC8 dynein light chain (DYNLL) is a hub protein, capable of binding proteins of various functions and influence their action. Being a dimeric protein with two equal binding sites it is thought to function as a dimerization engine. The structure of its complex with a high affinity binding peptide developed by in vitro evolutionary technique was determined by X-ray crystallography for characterizing specific interactions of the protein. In the complex the peptide was dimerized by a designed leuzine zipper in order to mimic the natural dimer binding to dimer mechanism and study possible distortion of the interactions in the dimer to dimer complex.
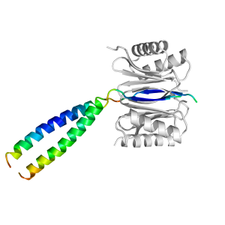
The structure of the DYNLL (grey) / LC8 (colored by B-factor) complex
Cooperation
Motor Proteins: Structural Biology Research Group of the Department of Biochemistry, Eötvös Loránd University, Budapest
Directed Evolution Research Group of the Department of Biochemistry, Eötvös Loránd University, Budapest
Structure determined (PDB code):
3P8M
Kapcsolódó publikációk
-
Péter Rapali , László Radnai , Dániel Süveges , Veronika Harmat , Ferenc Tölgyesi , Weixiao Y. Wahlgren , Gábor Pál
Directed evolution reveals the binding motif preference of the LC8/DYNLL hub protein and predicts large numbers of novel binders in the human proteome
PLoS One 6(4):e18818. | DOI: 10.1371/journal.pone.0018818 | PMID: 21533121 (2011) Kivonat