Synthesis of biologically active small molecules; potential inhibitors
Novel ferrocenyl-substituted thiazolones, 1,3-thiazines, pyrimidones and imidazolones were synthesized from acetyl-ferrocene by reactions with thiosemicarbazones and S-methyl derivatives and dimethyl acetylenedicarboxylate (Fig. 3). Synthesis of compounds containing ferrocene ring and variably functionalised aryl- and heteroaromatic molecules was accomplished and these were transformed to novel glycosidic- and oligopeptide derivatives. From amino-substituted ferrocenyl-phenyl-propenones preparation of new derivatives with steroid moiety was carried out. Some of the new compounds showed significant in vitro antitumor effect against human leukemia cells. Incorporation of the ferrocenyl group enhanced the biological activity favourably. Novel palladium complexes containing ferrocene moiety were synthesised and their catalytic activities were studied in various reactions, widely used in the industry.
For the purpose of biological investigations novel heteroaromatic compounds were prepared. For studying the ring-enlargement reactions of condensed tetra-cyclic compounds, tetrahydropyrazolo-benzodiazonin-8-one and pyrazolo-benzodiazocin-7-one derivatives were synthesized. Conformation, ring-inversion and kinetic parameters of that were investigated as function of the ring size and number of substituents. These results may contribute to a better understanding of the inherence between chemical reactivity and receptor binding properties of these molecules.
Structure determination of new heterocyclic compounds with potential antibacterial and anticancer activity was carried out applying a complex NMR investigation. These heteropolycyclic compounds containing 4-, 5- and 6-membered rings are formed in the reaction of 2-amino-1-aminomethyl-oxanorbornene with saturated and unsaturated 3-oxocarbonic acids possessing 1, 2 and 3 rings.
Structure elucidation of several new heterocycles with 1,3-thiazino[5,6-b]indole skeleton was carried out. These compounds are synthetic analogues of phytoalexins having antibacterial and anticancer effect.
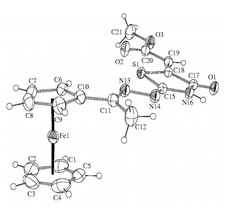
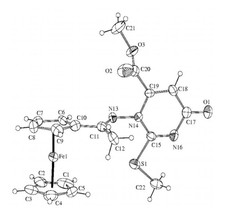
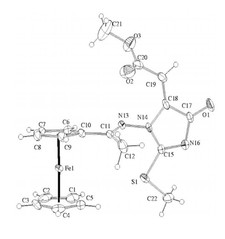
Figure 3.Crystal structure of novel ferrocenyl substituted compounds