Calmodulin-ligand complexes
Calmodulin (CaM) is a 148 residue long calcium-binding protein that plays a key role in signal transduction pathways of eukaryotic cells. The binding of Ca2+ ions induces large scale conformational changes of the protein which enables interaction with targets in a Ca2+-concentration dependent manner. The target peptides typically have a helical secondary structure, although neither their sequences, nor their anchoring points and binding orientations are not conserved. Variable binding modes depending on the segment length were reported.
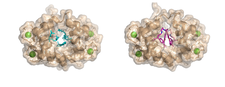
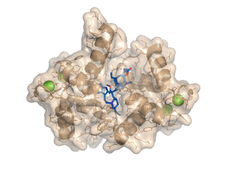
We studied complexes with small molecular ligands taking different effects on calmodulin action. Trifluoperazine and high affinity arlyalkylamine compounds (AAA) are competitive antagonists, binding to the hydrophobic pockets of both domains of CaM, the main anchoring sites of the majority of CaM binding target sequences. KAR-2, derivative of the anticancer drug Vinblastine, binds to CaM, but does not inhibit its action. Our crystallographic and NMR studies revealed, that KAR-2 binds in a region of CaM overlapping only partially with the binding sites of the target proteins. That explains why KAR-2 does not prevent CaM from binding most of its physiological targets. The lipid mediator molecule sphingosylphosphorylcholine and its micelles showed two-step binding mechanism to CaM.
The crystal structure shows that binding of few sphingolipid molecules occurs at the conventional hydrophobic binding region of CaM. The segments of the lipid molecules are disordered , located outside of this region (PDB codes:1a29, 1qiv, 1qiw, 1xa5, 3if7).
Kapcsolódó publikációk
-
István Horváth , Veronika Harmat , András Perczel , Villő Pálfi , László Nyitray , Attila Nagy , Emma Hlavanda , Gábor Náray-Szabó , Judit Ovádi
The structure of the complex of calmodulin with KAR-2: a novel mode of binding explains the unique pharmacology of the drug
J. Biol. Chem. 280(9):8266-8274. | PMID: 15596444 (2005) Kivonat -
Erika Kovács , Veronika Harmat , Judit Tóth , Beáta G. Vértessy , Károly Módos , József Kardos , Károly Liliom
Structure and mechanism of calmodulin binding to a signaling sphingolipid reveal new aspects of lipid-protein interactions
FASEB J. 24(10):3829-39. | DOI: 10.1096/fj.10-155614 | PMID: 20522785 (2010) Kivonat -
Veronika Harmat , Zsolt Böcskei , Gábor Náray-Szabó , Imre Bata , Andrea S. Csutor , István Hermecz , Péter Arányi , Beáta Szabó , Károly Liliom , Beáta G. Vértessy
A new potent calmodulin antagonist with arylalkylamine structure: crystallographic, spectroscopic and functional studies
J. Mol. Biol. 297(3):747-755. | DOI: 10.1006/jmbi.2000.3607 | PMID: 10731425 (2000) Kivonat -
Beáta G. Vértessy , Veronika Harmat , Gábor Náray-Szabó , Zsolt Böcskei , Ferenc Orosz , Judit Ovádi
Simultaneous binding of drugs with different chemical structures to Ca2+-calmodulin: crystallographic and spectroscopic studies
Biochemistry 37(44):15300-15310. | DOI: 10.1021/bi980795a | PMID: 9799490 (1998) Kivonat -
Zsolt Dürvanger , Veronika Harmat
Structural Diversity in Calmodulin - Peptide Interactions
Current Protein & Peptide Science 1102 - 1111 doi.org/10.2174/1389203720666190925101937 (2019) Kivonat