Structure of some sugar derivatives
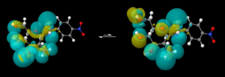
Cyclic or acyclic? Criteria determining the cyclic form of sugar arylhydrazones were studied. Buildup of the H-bonds and elements of stability were determined by using high resolution NMR and QM data. Selected structural properties of monosaccharide arylhydrazones were investigated. D-hexose arylhydrazones of different configurations, such as D-Gluco, D-Galacto, D-Manno, and D-Talo, were studied as well as new types of hexosamine phenylhydrazone s and 4-nitrophenylhydrazone derivatives were investigated.
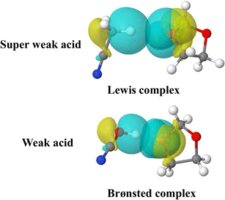
Complex formation ability and stability of both weak and super-weak acids were studied by mean of in silico determined thermodynamic data. While weak acids act like Brønsted acids forming H-bond type Brønsted complexes, super-weak acids rather form Lewis complexes via van der Waals interaction. Unlike in the former type, upon complexation, C-H distances changes insignificantly. Yet the complex formation is energy driven, ΔE zp < 0 kcal mol−1, which data support the Lewis complex formation, with the exception of CH4, an extremely “weak acid”.
Related articles:
Hydrogen-Bonding Network Anchors the Cyclic Form of Sugar Arylhydrazones
Viktória Goldschmidt Gőz , István Pintér , Antal Csámpai , Imre Jákli , Virág Zsoldos-Mády , András Perczel
Eur. J. Org. Chem. (2016) 20:3419-3426
How weak an acid can be? Variations of H-bond and/or van der Waals Interaction of Weak Acids
Szebasztián Szaniszló , Imre G. Csizmadia , András Perczel
Struct. Chem. (2017) 28(2):371-378.