Conformation analysis of foldamers
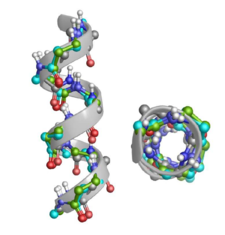
Foldamers often present either helical or extended like sheet structures depending on their building block properties. The secondary structure of these polypeptides can be determined by using various spectroscopic (ECD, VCD, NMR etc.) and X-ray diffraction data. The obtained experimental data are typically built in and interpreted by using various molecular modelling techniques such as MM, MD, QM/MM, ab initio etc. methods.
A systematic conformational search was carried out both for the monomers and homohexamers made form furanoid β-amino acids such as cis-(S,R) and trans-(S,S) isomers of aminocyclopentane carboxylic acid: ACPC, two different aminofuranuronic acids: AFUα and AFUβ, their isopropylidene derivatives: AFU(ip), and the key intermediate β-aminotetrahydrofurancarboxylic acid: ATFC as well.
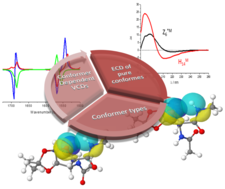
Different amide plane orientations, coupled to spectral changes, that are modest or on large scale can be tracked by electronic circular dichroism spectroscopy (ECD). The method introduced herein allows complete secondary structure assignment of foldamers, chimera, and so forth composed of common (α‐) and/or non‐proteinogenic amino acid residues of a given length.
Related articles:
Assignment of vibrational circular dichroism cross-referenced electronic circular dichroism spectra of flexible foldamer building blocks: towards assigning foldamers’ pure chiroptical properties
Viktor Farkas , Adrienn Nagy , Dóra K. Menyhárd , András Perczel
Chemistry A European Journal (2019) 25(65): 14890-14900 | doi.org/10.1002/chem.201903023 Kivonat
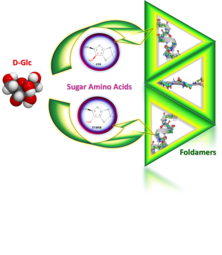
Predictable conformational diversity in foldamers of sugar amino acids
Dóra K. Menyhárd , Ilona Hudáky , Imre Jákli , György Juhász , András Perczel
J. Chem. Inf. Model. (2017) 57(4):757–768.