Interactions of Dynein Light Chain
A 10 kDa dynein light chain (DLC) has been shown to bind to the tail of myosin Va (myo5a). It is assumed that DLC functions as a cargo-binding and/or regulatory subunit of both motor proteins. NMR spectroscopy together with molecular docking simulations suggests that a short synthetic peptide of DBD binds to the surface grooves on DLC2, similarly to other known binding partners of DLCs. We hypothesize that DLC2 brings together the two DBDs of the myo5a heavy chains in an asymmetric manner, leaving one of the binding sites of the DLC2 dimer free to interact with a cargo or regulatory proteins.
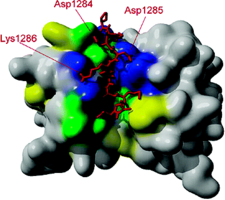
Binding of DLC to Myosin 5a
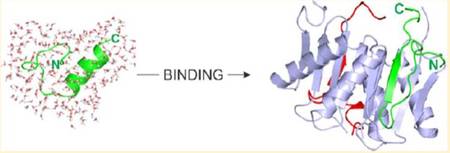
DLC2 binding region of myo5a was also investigated by NMR spectroscopy, X-ray crystallography, and MD simulations. In the free form, the DLC2 binding region - located in an intrinsically disordered domain of the myo5a tail - has a nascent helical character. The motif becomes structured and folds into a β-strand upon binding to DLC2. Interestingly, while the core motif shows a similar interaction pattern in the binding groove as seen in other complexes, the flanking residues make several additional contacts, thereby lengthening the binding motif. The involvement of flanking residues of the core binding site of myo5a can modify the quaternary structure of the full-length myo5a and affect its biological functions.
Cooperation with Dr. László Nyitray, Department of Biochemistry, Eötvös Loránd University, Budapest
Kapcsolódó publikációk
-
Zsuzsa Hódi , Attila L. Németh , László Radnai , Csaba Hetényi , Katalin Schlett , Andrea Bodor , András Perczel , László Nyitray
Alternatively spliced exon B of myosin Va is essential for binding the tail-associated light chain shared by dynein
Biochemistry 45:12582-12595. (2006) Kivonat -
Andrea Bodor , László Radnai , Csaba Hetényi , Péter Rapali , András Láng , Katalin E. Kövér , András Perczel , Weixiao Y. Wahlgren , Gergely Katona , László Nyitray
DYNLL2 Dynein Light Chain Binds to an Extended Linear Motif of Myosin 5a Tail That Has Structural Plasticity
Biochemistry 53(45):7107-7122. | DOI: 10.1021/bi500574z | PMID: 25312846 (2014) Kivonat