Ramachandran surface topology
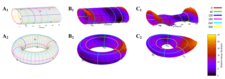
Folding the Ramachandran-surface coordinates first along φ (A1, cylindrical) and second along ψ (A2, toroidal) with minima highlighted, B1 and B2 are the same with color coded energy, C1 and C2 are energy enhanced surfaces.
The 2-torus representation of the 2D-Ramachandran map shows the correct continuity of the independent variables (e.g., φ, ψ) related to peptides and proteins backbone potential energy surface (PES). Such transformation of the coordinate system applied for the description of a typical peptide unit gives a more realistic and borderless view of the PES. In a 2-torus representation any point of the PES becomes embedded in its true environment and thus no artificial edges tumble their true topographic nature.
Torus viewer program free download: http://www.chem.elte.hu/departments/jimre/torus/

2D cross sections of the 4D Ramachandran map for the backbone of dihedral angles (φ, ψ) of the L- and D-Asn radicals.
Oxidative stress could initiate radical formation in proteins when e.g. the H-atom is abstracted from the Cα-carbon atom (e.g. C–H + •OH → C• + H2O). C-radicals have a trigonal planar structure and thus are achiral, regardless of whether they originate from a chiral or an achiral Cα-atom. Electronic structure calculations confirm that such a radical remains indeed achiral when formed from the achiral Gly, or the chiral Ala residue. However, when longer side-chain containing proteinogenic amino acid residues were studied (e.g. Asn), we found that radicals of axis chirality do form, which in turn leads to atropisomerism observed for the first time for peptides! (Atropisomers are stereoisomers arising because of hindered rotation about a single bond, and the energy differences create a barrier to rotation that is high enough to allow for isolation of individual conformers). The two enantiomeric extended backbone structures, •βL and •βD (monitored on a 4D-Ramachandran surface), have distinct transition states and indeed very different Gibbs-free energies.
Kapcsolódó publikációk
-
Jákli I , Knak Jensen SJ , Csizmadia IG , Perczel A
Variation of conformational properties at a glance. True graphical visualization of the Ramachandran surface topology as a periodic potential energy surface
CHEMICAL PHYSICS LETTERS 547: pp. 82-88. (2012) Kivonat -
Klára Z. Gerlei , Imre Jákli , Milán Szőri , Svend J. Knak Jensen , Béla Viskolcz , Imre G. Csizmadia , András Perczel
Atropisomerism of the Asn α Radicals Revealed by Ramachandran Surface Topology.
J. Phys. Chem. B 117(41):12402-12409. | DOI: 10.1021/jp4070906 | PMID: 24015919 (2013) Kivonat