Myosin regulation domain
Class II myosins have a fundamental role in muscle contraction and in a variety of cellular motilities. The regulatory domain (RD) acts as a lever arm during force generation, and it is the site of regulation in regulated conventional myosins. The class II myosin of Physarum polycephalum is uniquely inhibited by direct binding of Ca2+. In our structure of the regulatory domain, the regulatory Ca2+ is bound by the ELC. The Ca2+-binding first EF-hand unusually possesses "closed" conformation. In scallop myosin the activating Ca2+ is bound to the first EF-hand of the ELC that is also in a closed state, but it is coordinated by different residues. In the case of Physarum myosin II the change in dynamics of the molecule upon Ca2+-binding plays an important role in Ca2+-inhibition.
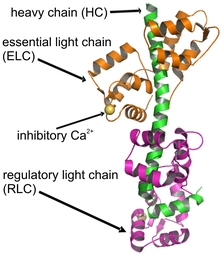
Structure of the myosin regulatory domain
Cooperation
Motor Proteins: Structural Biology Research Group of the Department of Biochemistry, Eötvös Loránd University, Budapest
Structures determined
Related publications
-
Judit É. Debreczeni , László Farkas , Veronika Harmat , Csaba Hetényi , István Hajdú , Péter Závodszky , László Nyitray , Kazuhiro Kohama
Structural evidence for non-canonical binding of Ca2+ to a canonical EF-hand of a conventional myosin
J. Biol. Chem. 280(50):41458-41464. | PMID: 16227209 (2005) Kivonat