Radiation damage
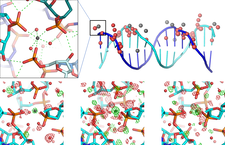
The advent of highly intense wiggler and undulator beamlines fed from synchrotron sources has reintroduced the age-old problem of X-ray radiation damage in macromolecular crystallography, even for crystals held at cryogenic temperatures (100 K). The high flux X-ray radiation induces photoelectric effect and causes radical reactions disrupting the order of crystal, lowering its diffraction power, and altering the proteins’ functional groups. While the sensitivity of protein crystals has been well characterized, crystals of DNA have not thus far been studied as thoroughly. In a collaboration with the Crystalography Lab of the Biological Research Centre, Hungarian Academy of Sciences, and Department of Biochemistry, University of Oxford a systematic investigation of radiation damage to a crystal of a DNA 16-mer up to an absorbed dose of 45 MGy, was carried out: the DNA crystal showed slightly lower radiation sensitivity to both global and specific damage, than protein crystals. Specific damage sites accumulated near bound calcium ions in the structure (PDB codes: 6qt1, 6qt2, 6qt3, 6qt4, 6qt5, 6qt6).
Related publications
-
Valéria Bugris , Veronika Harmat , Györgyi Ferenc , Sándor Brockhauser , Ian Carmichael , Elspeth F. Garman
Radiation-damage investigation of a DNA 16-mer
Journal of Synchrotron Radiation 998-1009 doi.org/10.1107/S160057751900763X (2019) Kivonat