Making foldamers
Both furanoid and pyranoid β-Sugar Amino Acids (Fmoc-RibAFU(ip)-OH, Fmoc-XylAFU(ip)-OH and Fmoc-GlcAPU(Me)-OH) were used as „Lego-elements”, to probe α- to β-, β- to β- and a β- to α-amino acid coupling conditions in chimera peptides.
Syntheses of oligomers of furanoid sugar amino acids (X), particularly α/β pentapeptides H-GG-X-GG-OH incorporating -RibAFU(ip)- or -XylAFU(ip)- as X were accomplished. The oligomers were synthesized on solid phase, using N3-X-OH, investigating with the Ph3P or the more reactive nBu3P reagent via the Staudinger reaction.
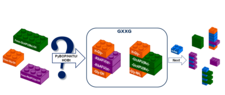
The fine-tuned solid phase synthesis of -GXXG- chimera peptides (X: Fmoc-RibAFU(ip)-OH and Fmoc-GlcAPU(Me)-OH) was completed. A comprehensive analysis was done to find the best coupling strategy and conditions, including the different coupling reagents, conditions assisted by time-resolved 1H-NMR spectroscopy.
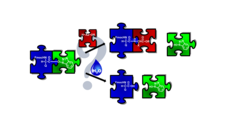
Nowadays, in Solid Phase Peptide Synthesis (SPPS) coupling is done via in situ active esters. Parallel to the α/β-amide bond formation the hydrolysis of active esters as unwanted side-reactions limiting coupling efficacy was monitored by time-resolved 1H NMR. A detailed kinetic analysis was conducted by using the most common PyBOP/DIEA and HOBt/DIC coupling reagents to couple both α- and β-amino acids.
Related publications:
Unwanted hydrolysis or α/β-peptide bond formation: how long should the rate-limiting coupling step take?
Viktória Goldschmidt Gőz , Adrienn Nagy , Viktor Farkas , Ernő Keszei , András Perczel
RSC Advances (2019), 9, 30720–30728
α/β‐Chimera peptide synthesis with cyclic β‐sugar amino acids: the efficient coupling protocol
Adrienn Nagy , Viktória Goldschmidt Gőz , István Pintér , Viktor Farkas , András Perczel
Amino Acids (2019) 51(4), 669-678
Origin of problems related to Staudinger reduction in carbopeptoid syntheses
Barbara Csordás , Adrienn Nagy , Veronika Harmat , Virág Zsoldos-Mády , Ibolya Leveles , István Pintér , Viktor Farkas , András Perczel
Amino Acids (2016) 48(11):2619-2633