Structure-function relationships of proteins
Calpastatin, an IUP is the inhibitor of the protease calpain involved in the regulation of cell division, differentiation and motility. Our NMR studies revealed that it binds to calpain with its three conserved motifs, which show weak preformed secondary structural elements in the free state of the protein. We have studied the roles of the conserved motifs and the flexible linker regions during the process calcium-dependent binding (Fig. 1). Our conclusions can be generalized for IUPs.
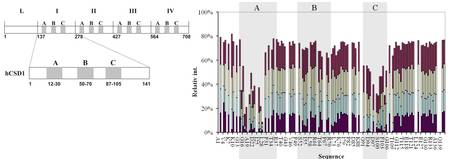
Figure 1. Left: Domain structure of human calpastatin with conserved regions A,B and C including motifs that bind to calpain. Right: NMR intensity loss of calpastatin indicates the regions interacting with calpain in our study of calcium dependent binding.
We studied the catalytic mechanism, function and regulation of proteases of the chymotrypsin family. Our ab initio calculations revealed that the pKa of the histidine of the catalytic triad depends predominantly on its conformation during the catalytic process. Enzymes MASP-1 and C1r are important elements of innate immunity. C1r catalyzes the first chemical reaction in a cascade leading to the elimination of the pathogen cell, rendering the mechanism of its autoactivation especially focused on. Based on our crystallographic studies we revisited the mechanism in more detail. Based on the crystal structure of MASP-1 we published a patent application. C1-inhibitor, the major down regulator of inflammatory processes in blood, is a regulator of C1r activity. The crystal structure solved may be of help to improve therapeutic C1-inhibitor preparations. We explained the molecular mechanism of the anti-inflammatory effect of heparin, which is based on its interaction with C1 inhibitor.
We characterized the interactions of prolyl oligopeptidase (a protease involved in memory and learning) and its novel nanomolar inhibitors. We solved the structures of their complexes and established structure-function relationships. Acylaminoacyl peptidase is a member of the prolyl oligopeptidase family. Our studies of the enzyme from Aeropyrum pernix K1 revealed its endopeptidase activity and identification of residues involved in substrate binding. We shed light on the role of the conserved His367: stabilizing the oxyanion site (Fig. 2).
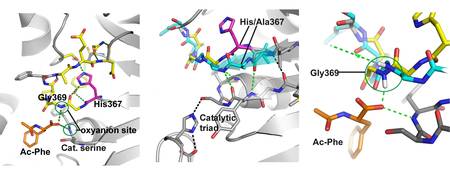
Figure 2. The role of His367 of acylaminoacyl hydrolases in stabilizing the oxyanion site. Left: His367 is stabilized by conserved contacts (yellow). Middle and right: superposition of the wild type enzym structure (yellow, magenta) and the His367Ala form (cyan) shows significant conformational change of the oxyanion site region.
The mechanism of phosphate ester hydrolysis catalyzed by dUTP-ase was explored by quantum chemical methods. We proved the existence of a trigonal bipyramidal intermediate, which suggests that the mechanism is associative. A retroviral dUTP-ase studied is a fusion protein which also contains a nucleocapsid domain. We studied the effect of this domain on the structure of the dUTP-ase trimer by using combined experimental methods.
The effect of the ligand on protein-ligand interactions was also studied: Antitumor activity of gonadotropin-releasing hormone variants from sea lamprey was studied. To establish structure-function relationships 3D-structures of designed peptides were characterized by taking a combined spectroscopic approach. GAT-1 maintains low resting synaptic level of gamma-aminobutyrate (GABA) hence it is a target of antiepileptic drugs. Using molecular modelling methods possible binding modes of GABA and its 12 derivatives were determined.