Membrane models and protein-membrane interactions
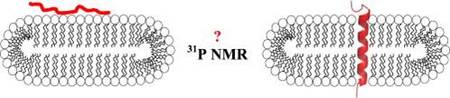
Small fast-tumbling bicelles are ideal models of studying membrane interactions at molecular level by solution NMR approaches. Using 31P-NMR relaxation measurements we obtain detailed information on lipid head-group dynamics.
We explored the effect of two topologically different membrane-interacting peptides on DMPC/DMPG neutral and DMPC/DMPG/DHPC negatively charged bicelles. KALP21 is a model transmembrane peptide, designed to span a DMPC bilayer and dynorphin B is a membrane surface active neuropeptide. Results indicate that the positively charged dynorphin B decreases the mobility of the lipid molecules -in particular for the negatively charged DMPG- while KALP21 has a more modest influence, which demonstrates that while a transmembrane peptide has severe effects on overall bilayer properties, the surface bound peptide has a more dramatic effect in reducing lipid head-group mobility.
We focused on changes in lipid dynamics upon interaction with the putative "paddle" domains from different K+-channels:KvAP (from Aeropyrum pernix) and HsapBK (human). 13C longitudinal relaxation time values and 13C-1H NOE data for DMPC in DMPC/DHPC bicelles and for DHPC in micelles showed that the lipid acyl chains in the bicelles become less flexible in the presence of either of the fragments. An even more pronounced effect is seen on the glycerol carbons. 2H NMR spectra of magnetically aligned bicelles showed that the peptide derived from KvAP had no or little effect on bilayer order, while the peptide derived from HsapBK had the effect of lowering the order of the bilayer.
We presented a combined NMR-SAXS method performed under physiological conditions, enabling to determine a global parameter, namely the hydration radius (rH) and estimating the bicelle shape. We use zwitterionic (DMPC/DHPC) and negatively charged (DMPC/DHPC/DMPG) bicelles and investigate the interaction with model transmembrane and surface active peptides (KALP23 and melittin). While the influence of the transmembrane KALP peptide is significant, effects upon addition of surface active melittin seem negligible. This synergy of techniques under controlled conditions can provide information about bicellar shape modulation occurring during peptide-bicelle interactions.
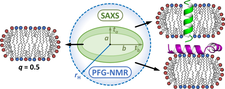
Combined NMR-SAXS approach for the characterization of peptide-bicelle interactions
Cooperation with Dr. Lena Mäler, Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden, Dr. Katalin Kövér, Department of Inorganic and Analytical Chemistry, University of Debrecen, Dr. Attila Bóta, Institute of Materials and Environmental Chemistry, Hungarian Academy of Sciences, Budapest, Hungary
We demonstrated how tartrazine (TZ), an anionic food additive commonly used in our diet, bind to DHVAR4, an antimicrobial peptide (AMP) derived from oral host defense peptides, resulting in significantly fostered toxic activity against both Gram-positive and Gram-negative bacteria, but not against mammalian cells.
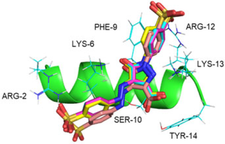
Binding of tartrazine to DHVAR4
Cooperation with Dr. Tamás Beke-Somfai, Institute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Budapest, Hungary
Kapcsolódó publikációk
-
Henrik Biverståhl , Jesper Lind , Andrea Bodor , Lena Mäler
Biophysical studies of the membrane location of the voltage-gated sensors in the HsapBK and KvAP K<sup>+</sup> channels.
Biochem. Biophys. Acta 1788:1976-1986 (2009) Kivonat -
Andrea Bodor , Katalin E. Kövér , Lena Mäler
Membrane interactions in small fast-tumbling bicelles as studied by 31P NMR
BBA Biomembranes 1848(3):760-766. | DOI: 10.1016/j.bbamem.2014.12.001 | PMID: 25497765 (2015) Kivonat -
Ádám Bartók , Krisztina Fehér , Andrea Bodor , Kinga Rákosi , Gábor K. Tóth , Katalin E. Kövér , György Panyi , Zoltán Varga
An engineered scorpion toxin analogue with improved Kv1.3 selectivity displays reduced conformational flexibility
Scientific Reports 5:18397. DOI: 10.1038/srep18397 | PMID: 26689143 (2015) Kivonat -
E. F. Dudás , A. Wacha , A. Bóta , A. Bodor
Peptide-bicelle interaction: Following variations in size and morphology by a combined NMR-SAXS approach
Biochimica et Biophysica Acta (BBA) - Biomembranes doi.org/10.1016/j.bbamem.2019.183095 (2020) Kivonat -
Maria Ricci , Kata Horváti , Tünde Juhász , Imola Szigyártó , György Török , Fanni Sebák , Andrea Bodor , László Homolya , Judit Henczkó , Bernadett Pályi , Tamás Mlinkó , Judith Mihály , Bilal Nizami , Zihuayuan Yang , Fengming Lin , Xiaolin Lu , Loránd Románszki , Attila Bóta , Zoltán Varga , Szilvia Bõsze , Ferenc Zsila , Tamás Beke-Somfai
Anionic food color tartrazine enhances antibacterial efficacy of histatin-derived peptide DHVAR4 by fine-tuning its membrane activity
Quarterly Reviews of Biophysics 53(5), 1-11 | DOI: 10.1017/S0033583520000013 (2020) Kivonat