Structures of foldamer building blocks and their precursors
Our research group is working on the synthesis of beta peptide foldamers, composed of sugar-amino acid building blocks. These are water-soluble, biocompatible and biodegradable materials of stable secondary structures. Crystal structures of the building blocks and precursors can explain their physical chemical properties like reactivity, preferred conformers and gel formation properties (CCDC reference codes: 1575872, 1922887,1922888).
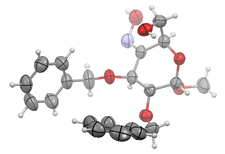
During the synthesis of alpha and beta peptides, using amino-furanuronic acid units, we discovered that the Staudinger reaction is unsuccessful for the cis-stereoisomers. Together with QM calculations and NMR studies, the crystal structure of N-methyl-3-deoxy-3-triphenylphosphiniminio-1,2-O-isopropylidene-alpha-D-xylofuranuronamide chloride helped to understand the unexpected stability of this intermediate, due to steric crowding and electronic effects. The crystal structure of methyl 2,3-di-O-benzyl-alpha-D-xylopyranoside-4-ulose oxime, a precursor of an amino-pyranuronic acid building block was also solved. Analysis of H-bonding network revealed the possible direction of protonation of the oxime group, prior the reaction.
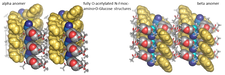
The self-association properties of the alpha and beta anomers of O-Ac,N-Fmoc-D-Glucosamine are very different. While the alpha-anomer readily crystallizes from solution, the beta-anomer forms gel phases at various conditions. Crystals form from the gel phase as well. For exploring the conformation and interaction characteristics of these molecules, along with other methods, their crystal structures were analyzed. Though both anomers form linear N-H..O hydrogen bonded network of molecules in extended conformation, the crystal structure of the beta-anomer forms a more loosely associated superstructure of otherwise well-packed 2D-layers. Interestingly, something similar happens in case of aggregation and amyloid formation of polypeptides and proteins.
Kapcsolódó publikációk
-
Barbara Csordás , Adrienn Nagy , Veronika Harmat , Virág Zsoldos-Mády , Ibolya Leveles , István Pintér , Viktor Farkas , András Perczel
Origin of problems related to Staudinger reduction in carbopeptoid syntheses
Amino Acids 48(11):2619-2633 DOI: 10.1007/s00726-016-2289-x | PMID: 27438266 (2016) Kivonat -
Viktória Goldschmidt Gőz , István Pintér , Veronika Harmat , András Perczel
Approaches to Pyranuronic beta-Sugar Amino Acid Building Blocks of Peptidosaccharide Foldamers
Eur. J. Org. Chem. 355-361. DOI: 10.1002/ejoc.201701612 (2018) Kivonat -
Anita Kapros , Attila Balázs , Veronika Harmat , Adrienn Háló , Lívia Budai , István Pintér , Dóra K. Menyhárd , András Perczel
Configuration‐Controlled Crystal and/or Gel Formation of Protected d‐Glucosamines Supported by Promiscuous Interaction Surfaces and a Conformationally Heterogeneous Solution State
Chemistry—A European Journal 26 | doi.org/10.1002/chem.202000882 (2020) Kivonat