Published December 20, 2024 14:23
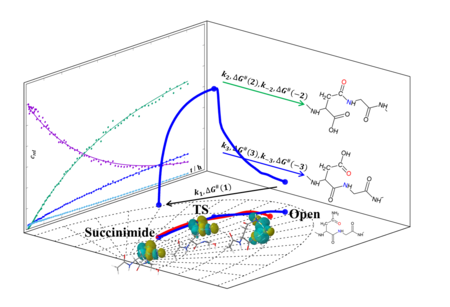
In a recent article, we investigated the spontaneous isomerization reaction of peptides containing the asparagine-glycine (-NG-) sequence as a function of pH, temperature and chemical composition (Ac-NGXA-NH2 (X = K(+): lysine, R(+): arginine, A: alanine or E(-): glutamic acid)). We have presented a kinetic and thermodynamic model based on quantitative NMR data in order to obtain a coherent and quantitative description of the transformation. The rate-determining step of isomerization, which is the deamidation and ring closure of the -NG- motif, was investigated in more detail by IRC and NBO analysis. By the end of the ring closure, four to six key intermediate structures were identified and the charge and structural parameters of the transformation were determined.