Study of amyloidogenic polypeptides
Amyloid fibrils are associated with a number of human diseases, including Alzheimer’s and Huntington’s disease, Type II Diabetes Mellitus and prion diseases. Despite their different protein precursors all share a common structural characteristic, namely the ‘cross-β spine’. Our studies aim to explore whether Cys residue pairs in polypeptide strands can stabilize (in the thermodynamic sense) β-sheets formation. The model (containing two strands of chiral amino acid residues) allows the possibility to form either a single or double inter- or intra-strand disulfide bridges and to assess the stability of β-strand foldamers as function of disulfide bridges and their oxidation states via a QM based description, heading to explain the intrinsic feature of polypeptide aggregation.
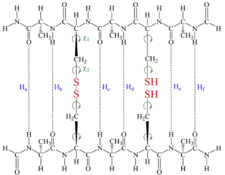
Partially oxidized form of the Ala-Cys-Ala-Cys-Ala model system
The 20 residue long Trp-cage miniprotein is a great model to complete both computational and experimental studies on protein folding and stability. In addition, Trp-cage is suitable for modeling aggregation and amyloid formation. We characterized the amyloid formation of several sequence modified and side-chain phosphorylated Trp-cage variants. We demonstrated that the native fold is destabilized upon serine phosphorylation, and the resultant highly dynamic structures form amyloid-like ordered aggregates with high intermolecular β-structure content. It was found that the optimum temperature for E5 variant of the Trp-cage miniprotein amyloidosis coincides with body temperature and requires well below physiological salt concentration. Results indicate that the amyloid transition is triggered by subtle misfolding of the α‐helix exposing aromatic and hydrophobic side chains, that may provide the first centers for an intermolecular reorganization. These initial clusters provide the spatial closeness and sufficient time for a transition to the β‐structured amyloid nucleus thus the process follows a nucleated growth mechanism. We showed that Trp-cage miniprotein and its variants are indeed a realistic model of larger globular systems of composite folding and aggregation landscapes and helps us to understand the fundamentals of protein aggregation and amyloid formation.
Related publications
-
Natalie J. Galant , Heeyeon Cheryl Song , Imre Jákli , Béla Viskolcz , Imre G. Csizmadia , Svend J. Knak Jensen , András Perczel
A theoretical study of the stability of disulfide bridges in various β-sheet structures of protein segment models
Chem. Phys. Lett. 593:48–54. | DOI: 10.1016/j.cplett.2013.12.065 (2014) Kivonat -
József Kardos , Bence Kiss , András Micsonai , Petra Rovó , Dóra K. Menyhárd , János Kovács , Gábor K. Tóth , András Perczel
Phosphorylation as Conformational Switch from the Native to Amyloid State: Trp-Cage as a Protein Aggregation Model
J. Phys. Chem. B 119(7):2946–2955. | DOI: 10.1021/jp5124234 | PMID: 25625571 (2015) Kivonat -
Nóra Taricska , Dániel Horváth , Dóra K. Menyhárd , Hanna Ákontz-Kiss , Masahiro Noji , Masatomo So , Yuji Goto , Toshimichi Fujiwara , András Perczel
The route from the folded to the amyloid state: exploring the potential energy surface of a drug-like miniprotein
Chemistry A European Journal 26(9), 1968-1978 | doi.org/10.1002/chem.201903826 (2020) Kivonat