Published August 30, 2013 12:35
Foldamer building block structure confirmed by low temperature MI-IR spectroscopy (Pohl et al. Amino Acids. 2013 July)
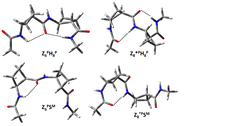
In cooperation with György Tarczay (ELTE) and Rosa Ortuno (Barcelona) local chirality driven conformational preferences of small beta-peptides, X-(ACBA) n -Y, were determined by matrix-isolation IR spectroscopy. For the very first time selected cyclic-beta residues formed oligopeptides were deposited in a frozen (~10 K) noble gas matrix. The recorded sharp vibrational lines were analyzed with QM data and foldamer structures were confirmed, used in the future to stabilize flexible parts of proteins.