Synthesis of β-Sugar amino acids (β-SAAs)
β-sugar amino acids (β-SAAs) are biocompatible and tunable building blocks of foldamers and α/β chimera peptides. Preparation of furanoid (e.g. Fmoc-RibAFU(ip)-OH and its C3-epimer, Fmoc-XylAFU(ip)-OH), as well as pyranoid residues (e.g. Fmoc-GlcAPU(Me)-OH and its C4-epimer Fmoc-GalAPU(Me)-OH) was completed. We currently work on efficient and economical synthetic pathways, focusing on higher yields and greener approaches making ten gram quantities for solid phase polypeptide synthesis.
The syntheses of Fmoc-RibAFU(ip)-OH and Fmoc-XylAFU(ip)-OH from D-glucose was accomplished in 8 and 9 consecutive steps. The sulfonate→azide replacement was conducted by using various 3-O-sulfonyl derivatives, such as the triflate, the mesylate, the tosylate, and the imidazylate to find the best conditions. The carboxyl function was obtained in a two-step oxidation via the appropriate aldehyde.
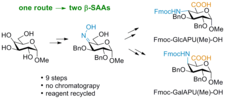
The pyranoid β-SAAs, both FmocGlcAPU(Me)-OH and Fmoc-GalAPU(Me)-OH, were prepared via a common oxime intermediate, using non-selective reduction method: 9 consecutive steps, starting from methyl α-D-glucopyranoside.
These β-SAAs are now used to synthesize both β-peptides and α/β-chimera peptides. Both H-RibAFU(ip)-NHMe and its precursor N3-RibAFU(ip)-OH were coupled in solution to make the first β-dipeptide of this kind, followed by the solid phase synthesis of α/β pentapeptides H-Gly-Gly-X-Gly-Gly-OH as test models. (HIV)
Related publications:
Approaches to Pyranuronic beta-Sugar Amino Acid Building Blocks of Peptidosaccharide Foldamers,
Viktória Goldschmidt Gőz , István Pintér , Veronika Harmat , András Perczel,
Eur. J. Org. Chem. (2018) 355-361
C‐3 epimers of sugar amino acids as foldameric building blocks: improved synthesis, useful derivatives, coupling strategies
Adrienn Nagy , Barbara Csordás , Virág Zsoldos-Mády , István Pintér , Viktor Farkas , András Perczel,
Amino Acids (2017) 49(2):223–240
Origin of problems related to Staudinger reduction in carbopeptoid syntheses
Barbara Csordás , Adrienn Nagy , Veronika Harmat , Virág Zsoldos-Mády , Ibolya Leveles , István Pintér , Viktor Farkas , András Perczel
Amino Acids (2016) 48(11):2619-2633