Characterization of intrinsically disordered proteins and their interactions
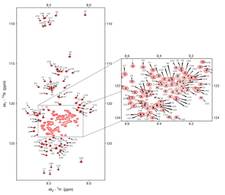
Calpastatin is a functionally disordered inhibitor of the intracellular calcium-regulated cysteine protease calpain. Using chemical shift mapping and relaxation studies we have shown that the functional segments of calpastatin exhibit characteristic local structural preferences with reduced mobility. Calpain binding is tripartite with flexible linkers between the contact point allowing high speed and specificity of target binding.
Summary of NMR analysis of the intrinsic flexibility of calpastatin
The plant dehydrin ERD14 is a functionally disordered stress protein expressed during dehydration. Chemical shift and relaxation analysis revealed that this 185 residue long protein is fully disordered under native conditions with short regions of slight helical propensity, suggested to correspond to preformed structural elements indispensable for biological function.
Thymosine β4 (Tβ4) is a 43 amino acid long intrinsically disordered protein (IDP), and its 4 described partners, PINCH, ILK, and stabilin-2, and G-actin, are structurally unrelated (the CTD of stabilin-2 is actually fully disordered), it occurred to us that this system might be ideal to characterize the structural adaptability and ensuing moonlighting functions of IDPs using NMR along with several other techniques.
A fragment of myosin Va (myo5a), which is intrinsically disordered as well, was investigated by NMR spectroscopy, X-ray crystallography, and MD simulations. In the free form, the dynein light chain (DLC2) binding region, located in an intrinsically disordered domain of the myo5a tail, has a nascent helical character. The motif becomes structured and folds into a β-strand upon binding to DLC2 showing a disorder-to-order transition. Interestingly, while the core motif shows a similar interaction pattern in the binding groove as seen in other complexes, the flanking residues make several additional contacts, thereby lengthening the binding motif. The N-terminal extension folds back and partially blocks the free edge of the β-sheet formed by the binding motif itself.
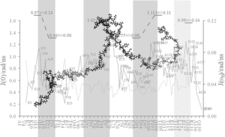
The fragment from binding region of nonmuscle-myosin IIA (MPT) to S100A4 is also intrinsically disordered which has a nascent helicity shown by SSP (secondary structure propensity) values. This nascent helicity was evidenced by relaxation measurements used for reduced spectral density analysis too. In this case the disorder-to-order transition of the entire fragment results in a substantial helix when interacting with S100A4 as shown by NMR and X-ray structures.
We characterized several other intrinsically disordered protein regions, e.g. TPPP/p25, RSK1 protein fragments and DF31.
Cooperation with
Dr. László Nyitray, Department of Biochemistry, Eötvös Loránd University, Budapest,
Dr. Attila Reményi, Institute of Enzymology, Biological Research Center, Hungarian Academy of Sciences, Budapest
Dr. Judit Ovádi, Institute of Enzymology, Biological Research Center, Hungarian Academy of Sciences, Budapest
Cooperation with Dr. Péter Tompa, Laboratory of Intrinsically Disordered Proteins, Institute of Enzymology, Budapest
Related publications
-
Róbert Kiss , Dénes Kovács , Péter Tompa , András Perczel
Local structural preferences of calpastatin, the intrinsically unstructured protein inhibitor of calpain
Biochemistry 47:6936-6945. (2008) Kivonat -
Róbert Kiss , Zoltán Bozóky , Dénes Kovács , Gergely Róna , Péter Friedrich , Péter Dvortsák , Rudinger Weisemann , Péter Tompa , András Perczel
Calcium-induced tripartite binding of intrinsically disordered calpastatin to its cognate enzyme, calpain
FEBS Lett. 582:2149-2154. (2008) Kivonat -
Szalainé Ágoston B , Kovács D , Tompa P , Perczel A
Full backbone assignment and dynamics of the intrinsically disordered dehydrin ERD14.
BIOMOLECULAR NMR ASSIGNMENTS 5:(2) pp. 189-193. (2011) Kivonat -
Zoltán Gáspári , Annamária F. Ángyán , Somdutta Dhir , Dino Franklin , András Perczel , Alessandro Pintar , Sándor Pongor
Probing dynamic protein ensembles with atomic proximity measures
Curr. Protein Pept. Sci. 11(7):515-522. | DOI: 10.2174/138920310794109201 | PMID: 20887264 (2010) Kivonat -
Edit Szőllősi , Mónika Bokor , Andrea Bodor , András Perczel , Éva Klement , Katalin F. Medzihraszky , Kálmán Tompa , Péter Tompa
Intrinsic structural disorder of DF31, a Drosophila protein of chromatin decondensation and remodeling activities
J. Proteome Res. 7:2291-2299. (2008) Kivonat -
Zotter A , Oláh J , Hlavanda E , Bodor A , Perczel A , Szigeti K , Fidy J , Ovádi J
Zn(2+)-induced rearrangement of the disordered TPPP/p25 affects its microtubule assembly and GTPase activity.
BIOCHEMISTRY 50:(44) pp. 9568-9578. (2011) Kivonat -
Ágnes Tantos , Beáta Szabó , András Láng , Zoltán Varga , Maksym Tsylonok , Mónika Bokor , Tamás Verebélyi , Pawel Kamasa , Kálmán Tompa , András Perczel , László Buday , Si Hyung Lee , Yejin Choo , Kyou-Hoon Han , Péter Tompa
Multiple fuzzy interactions in the moonlighting function of thymosin-β4
Intrinsically Disordered Proteins (2013) Kivonat -
Andrea Bodor , László Radnai , Csaba Hetényi , Péter Rapali , András Láng , Katalin E. Kövér , András Perczel , Weixiao Y. Wahlgren , Gergely Katona , László Nyitray
DYNLL2 Dynein Light Chain Binds to an Extended Linear Motif of Myosin 5a Tail That Has Structural Plasticity
Biochemistry 53(45):7107-7122. | DOI: 10.1021/bi500574z | PMID: 25312846 (2014) Kivonat -
Anita Alexa , Gergő Gógl , Gábor Glatz , Ágnes Garai , András Zeke , János Varga , Erika Dudás , Norbert Jeszenői , Andrea Bodor , Csaba Hetényi , Attila Reményi
Structural assembly of the signaling competent ERK2–RSK1 heterodimeric protein kinase complex
Proc. Natl. Acad. Sci. USA 112(9):2711-2716. | DOI: 10.1073/pnas.1417571112 | PMID: 25730857 (2015) Kivonat -
Gyula Pálfy , Bence Kiss , László Nyitray , Andrea Bodor
Multi-level changes in protein dynamics upon complex formation of the calcium-loaded S100A4 with a non-muscle myosin IIA tail fragment
ChemBioChem 17(19):1829-1838 DOI: 10.1002/cbic.201600280 | PMID: 27418229 (2016) Kivonat -
Klára Z. Gerlei , Imre Jákli , Milán Szőri , Svend J. Knak Jensen , Béla Viskolcz , Imre G. Csizmadia , András Perczel
Atropisomerism of the Asn α Radicals Revealed by Ramachandran Surface Topology.
J. Phys. Chem. B 117(41):12402-12409. | DOI: 10.1021/jp4070906 | PMID: 24015919 (2013) Kivonat -
Beáta Biri-Kovács , Bence Kiss , Henrietta Vadászi , Gergő Gógl , Gyula Pálfy , György Török , László Homolya , Andrea Bodor , László Nyitray
Ezrin interacts with S100A4 via both its N- and C-terminal domains
PLoS One 12(5):e0177489 | doi: 10.1371/journal.pone.0177489 | PMID: 28493957 (2017) Kivonat -
Péter Ecsédi , Neil Billington , Gyula Pálfy , László Nyitray , Bence Kiss , Éva Bulyáki , Andrea Bodor , James R. Sellers
Multiple S100 protein isoforms and C-terminal phosphorylation contribute to the paralog-selective regulation of nonmuscle myosin 2 filaments
The Journal of Biological Chemistry 293(38) 14850 –14867. | DOI10.1074/jbc.RA118.004277 (2018) Kivonat -
Gergő Gógl , Anita Alexa , Bence Kiss , Gergely Katona , Mihály Kovács , Andrea Bodor , Attila Reményi , László Nyitray
Structural basis of Ribosomal S6 Kinase 1 (RSK1) inhibition by S100B Protein: modulation of the Extracellular Signal-regulated Kinase (ERK) signaling cascade in a calcium-dependent way
J. Biol. Chem. 291(1):11-27. DOI: 10.1074/jbc.M115.684928 | PMID: 26527685 (2016) Kivonat