Preparative and synthetic laboratory
Gallery: Preparative and synthetic laboratory
Facility manager: Viktória Goldschmidt Gőz, PhD
Members: Dr. Viktor Farkas, Duong Kim Hoang Yen, Kristóf Ferentzi, Elina Sarolta Lajtos , Dr. István Pintér, Szebasztián Szaniszló , Gergő Hegedűs, Gréta Nagy
By using modern organic chemistry methods we are synthesising different carbohydrate derivatives, beta amino acid residues, oligo- and polypeptides and chimeras. The total synthesis and the condensation reactions of both hydrophilic and hydrophobic, five- and six-membered ring containing amino acids are completed. Working on these monomers both cost efficiency and the possibility to scale up the synthetic method are among our goals. The optically active building units are used for the synthesis of foldamers.
Laboratory News
-
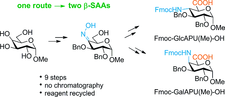
Approaches to Pyranuronic β-Sugar Amino Acid Building Blocks of Peptidosaccharide Foldamers
Pyranuronic β-sugar amino acids (β-SAAs) are biocompatible and tuneable building blocks of foldamers and α/β chimera peptides. The scalable and economical total synthesis of two building blocks was described. These C-4 epimers, Fmoc-GlcAPU(Me)-OH and Fmoc-GalAPU(Me)-OH, which are suitable for solid phase peptide synthesis, were prepared via a common oxime intermediate. The new synthesis uses nine consecutive steps, starting from methyl α-D-glucopyranoside. The synthesis is fine-tuned, optimized, and ready for largescale and cost-efficient production.
Eur. J. Org. Chem. 2018, 355
-
New building blocks synthesized: just published in Amino Acids
„C-3 epimers of sugar amino acids as foldameric building blocks: improved synthesis, useful derivatives, coupling strategies”. Yield optimized, scalable and environment-friendly protocols for making β-sugar amino acids and their derivatives to built up α/β-chimera peptides.
.jpg)
-
More news from the lab.
Our recent paper in Amino Acids explains why the application of Ph3P as Staudinger reagent is "ineffective" in the case of sterically hindered azido-compounds during solid phase peptide syntheses.
-
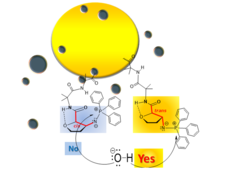
E.Fischer's 100 years old question answered on arylhydrazone stability
Our paper, Hydrogen-Bonding Network Anchors the Cyclic Form of Sugar Arylhydrazones (DOI: 10.1002/ejoc.201600462), has been accepted in EurJOC. Congratulations Viki!
Publications
-
Viktória Goldschmidt Gőz , István Pintér , Antal Csámpai , Imre Jákli , Virág Zsoldos-Mády , András Perczel
Hydrogen-Bonding Network Anchors the Cyclic Form of Sugar Arylhydrazones
Eur. J. Org. Chem. 20:3419-3426. DOI: 10.1002/ejoc.201600462 (2016) Kivonat -
Adrienn Nagy , Barbara Csordás , Virág Zsoldos-Mády , István Pintér , Viktor Farkas , András Perczel
C‐3 epimers of sugar amino acids as foldameric building blocks: improved synthesis, useful derivatives, coupling strategies
Amino Acids 49(2):223–240. | doi: 10.1007/s00726-016-2346-5 | PMID: 27803987 (2017) Kivonat -
Viktória Goldschmidt Gőz , István Pintér , Veronika Harmat , András Perczel
Approaches to Pyranuronic beta-Sugar Amino Acid Building Blocks of Peptidosaccharide Foldamers
Eur. J. Org. Chem. 355-361. DOI: 10.1002/ejoc.201701612 (2018) Kivonat -
Viktória Goldschmidt Gőz , Adrienn Nagy , Viktor Farkas , Ernő Keszei , András Perczel
Unwanted hydrolysis or α/β-peptide bond formation: how long should the rate-limiting coupling step take?
RSC Advances 9(53): 30720–30728 | DOI: 10.1039/c9ra06124j (2019) Kivonat