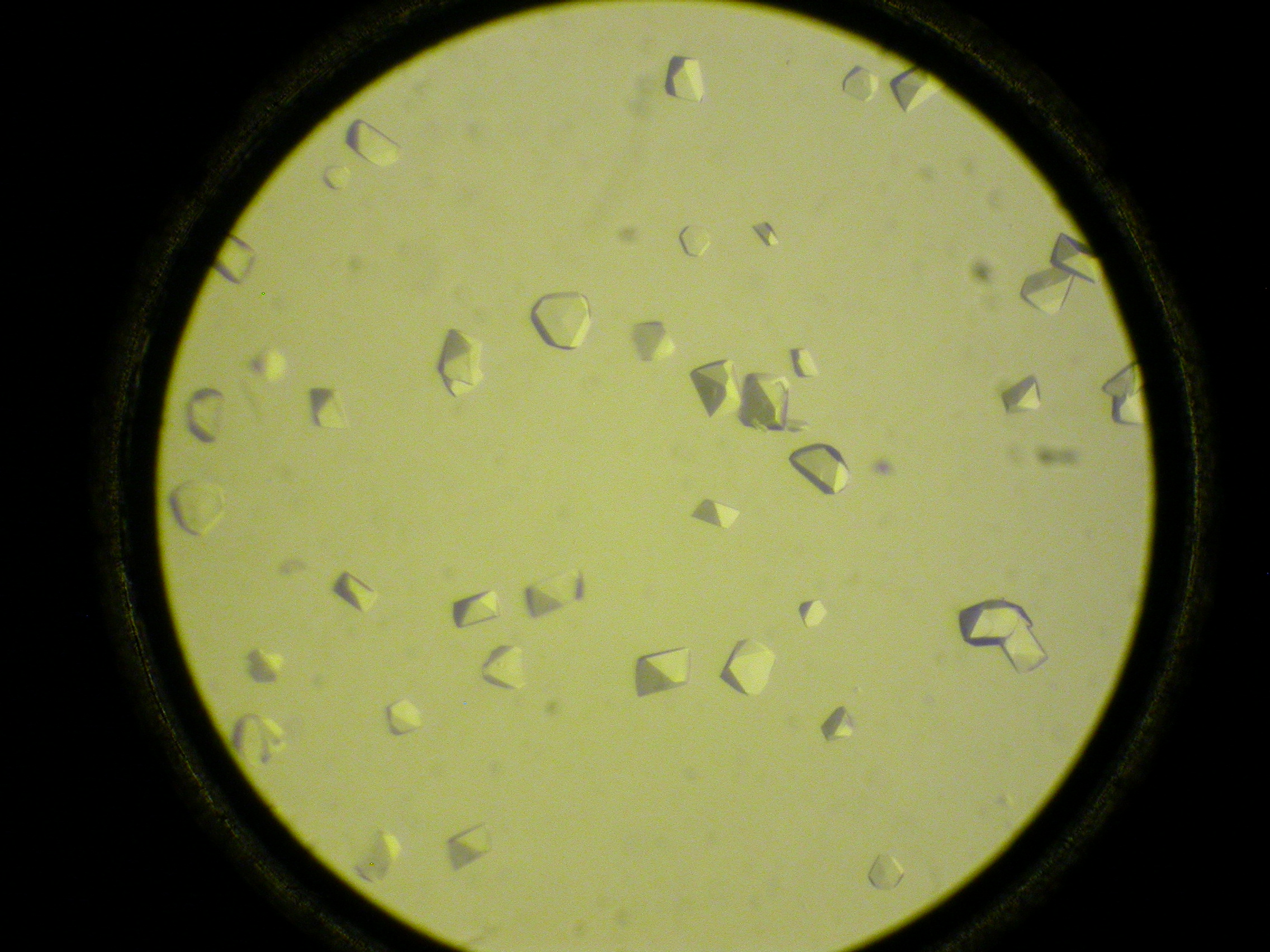
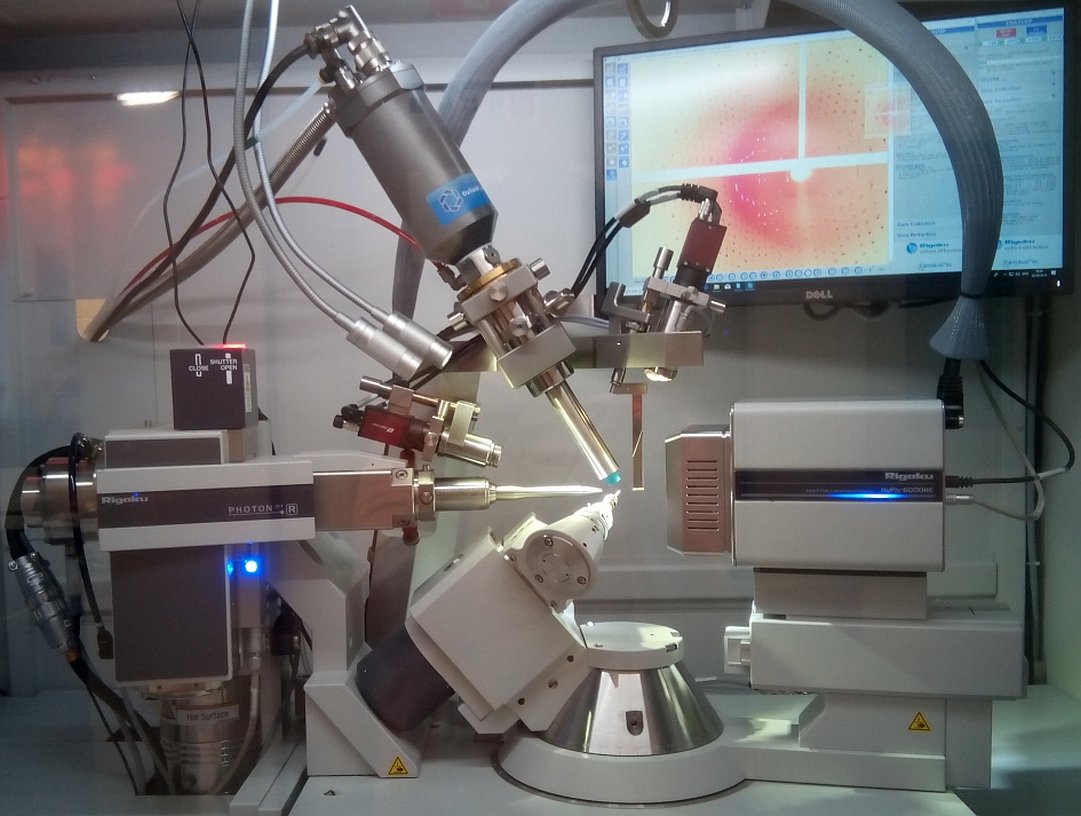
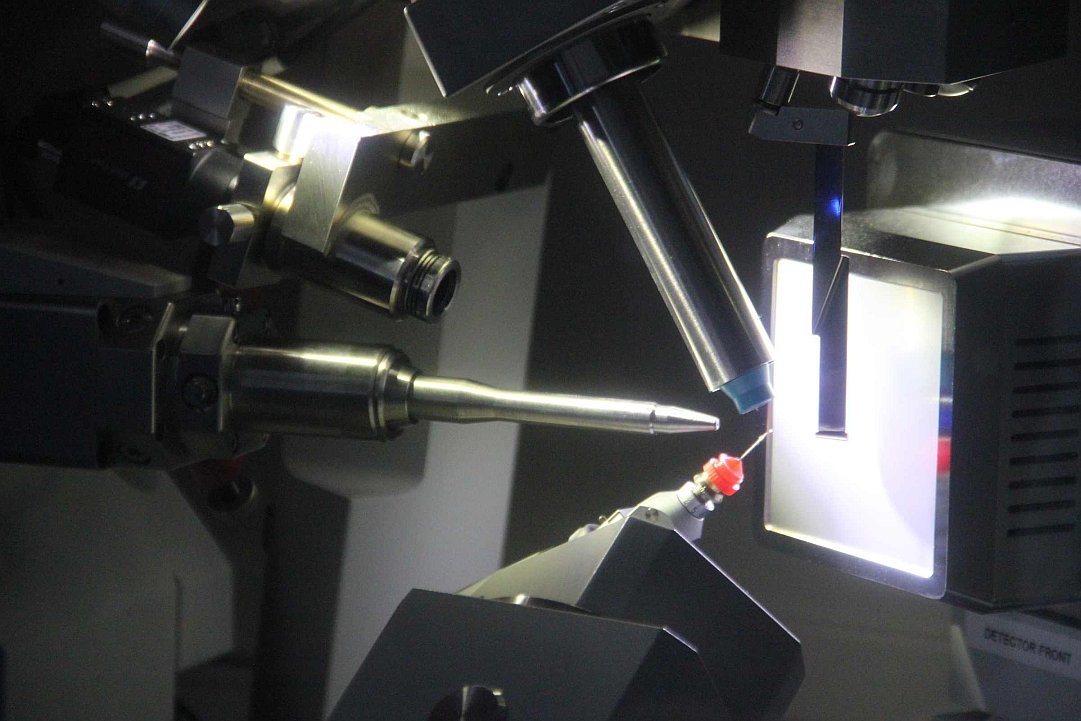
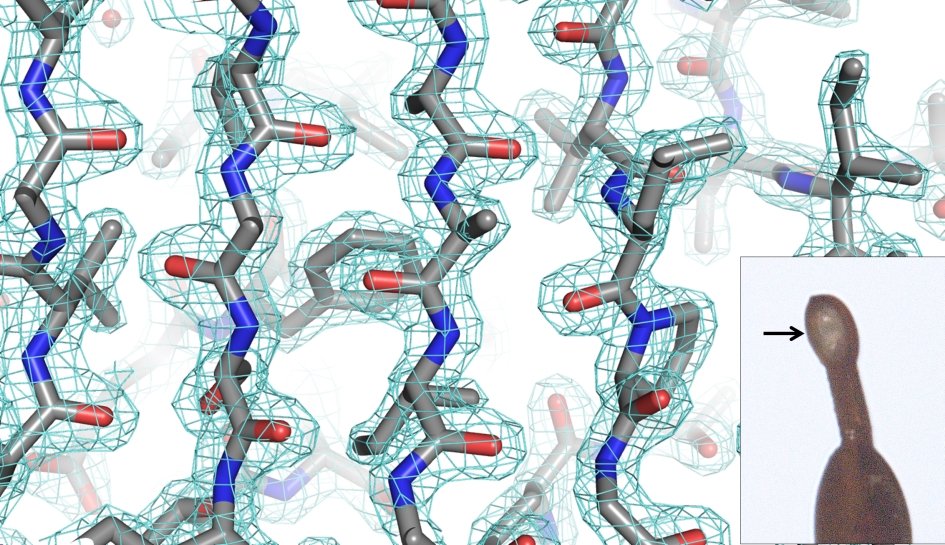
Protein crystallography lab.
Gallery: Protein crystallography lab.
Facility manager: Dr. Veronika Harmat
Members: Csaba Hegedűs, Dr. Zsolt Dürvanger, István Bódy, Dr. Veronika Harmat, Dr. Dóra K. Menyhárd , Máté Sulyok-Eiler , Anna J. Kiss-Szemán, PhD
For a better understanding of function and interactions of biological macromolecules, 3D-structure is required at an atomic resolution, typically determined by protein crystallography; a perfect method of choice. On the other hand, small molecular crystallographic studies explore the structural basis of physical-chemical characteristics of the compound. In 2018. new X-ray crystallographic instruments were installed in the EU 2020 framework (Széchenyi 2020*). The aim of these consortial projects is to establish an infrastructure serving as an open lab for a wider range of research groups (ELTE-CrystalLAB). The Rigaku XtaLAB Synergy-R X-ray diffractometer is capable for small molecular and protein crystallographic data collection as well as in situ crystal testing. A TTP Labtech Mosquito-LCP is available for automated crystallization, and monitoring crystal growth is carried out by a Formulatrix Rock Imager 2 system.
*The research within project No. VEKOP-2.3.3-15-2017-00018 and VEKOP-2-3-2-16-2017-00014 was supported by the European Union and the State of Hungary, co-financed by the European Regional Development Fund.
Laboratory News
-
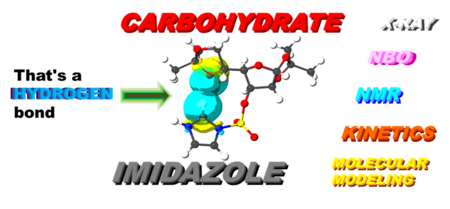
Intramolecular Inhibition by Imidazole in Acid-Catalyzed Hydrolysis of Protected Carbohydrates
The study uncovers an anomaly in the acid-catalyzed hydrolysis of the 5,6-O-isopropylidene group in specific D-gluco- and D-allofuranose derivatives, linked to C3-epimers with 3-O-imidazole sulfonyl moieties. Advanced modeling reveals how interactions between protecting groups and steric effects inhibit this reaction, providing new insights into carbohydrate chemistry.
-
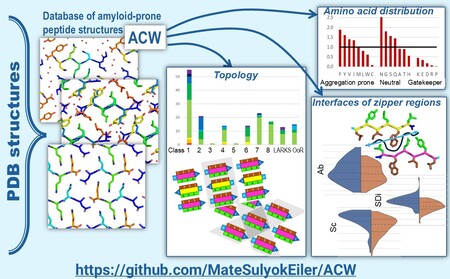
ACW: Database and toolkit for analyzing the zipper regions of amyloid oligopeptide structures
Amyloidogenic proteins contain short segments (amyloid prone regions) that nucleate formation of large sandwiched β-sheet structures. Analysis of structural properties of interfaces formed between the interacting β-sheets of these oligopeptides is essential for understanding their amyloidogenicity and self-assembly. We developed a tool (ACW plugin for the PyMOL program) for visualization and comparative analysis of amyloid oligopeptide crystal structures and applied it to the database of amyloid oligopeptides derived from the PDB. Our data show that parallel β-sheet structures have higher entanglement and better shape complementarity, probably related to their higher abundance. Our paper was published in the Journal of Chemical Information and Modeling.
-
-
ELTE-CrystalLAB: Interactive program at the Hungarian "Researcher’s Night” event
We participated in the Hungarian popular science event "Researcher’s Night" with a hands on demo lab entitled "Tales about crystals". (In Hungarian).
-
Amyloidogenic hexapeptides
In our latest article we studied amyloidogenic hexapeptides in solution and crystalline phases. We have shown that small modifications of the experimental conditions can result in extraordinarily diverse amyloid structures. These results indicate that amyloid polymorphism, which plays impotant role in the biologly of amyloids, can be modelled effectively with short peptides.
-
Polymorphic amyloid nanostructures of hormone peptides involved in glucose homeostasis display reversible amyloid formation
A group of hormone peptides is stored in the form of functional amyloid fibers in acid secretion vesicles before they reassemble their biologically active helical spatial structure when they enter the blood circulation. In the article published in Nature Communications, we explain the molecular basis of pH-dependent reversible amyloid formation in the case of gastrointestinal peptides belonging to the glucagon family. The article is presented on the website of the MTA by several Hungarian online newspapers. [1, 2, 3]
-
High affinity binding in a flexible protein-protein complex: structural studies on the calmodulin – melittin complex
Interaction of calmodulin and melittin is widely used as a test system for method development, and as a reference in studies of calmodulin interactions. However, results on the interaction pattern of this complex were non-conclusive so far. Our aim was to explore the fine details of this interaction. Our crystallographic study revealed multiple different binding modes, and our succeeding MD simulations concluded that these could transform to each other: the nanomolar affinity binding of this complex is realized by a ‘slippery’ binding way and interchanging interactions.
Our publication has been selected as one of "Editors' Picks," of the Journal of Biological Chemistry. -
-
Publications
-
Veronika Harmat , Klarissza Domokos , Dóra K. Menyhárd , Anna Palló , Zoltán Szeltner , Ilona Szamosi , Tamás Beke , Gábor Náray-Szabó , László Polgár
Structure and catalysis of acylamonoacyl peptidase: closed and open subunits of a dimer oligopeptidase
Journal of Biological Chemistry 286:1987-1998. PMID: 21084296 | DOI: 10.1074/jbc.M110.169862 (2011) Kivonat -
József Dobó , Veronika Harmat , László Beinrohr , Edina Sebestyén , Péter Závodszky , Péter Gál
MASP-1, a Promiscuous Complement Protease: Structure of Its Catalytic Region Reveals the Basis of Its Broad Specificity
J. Immunol. 183:1207-1214. (2009) Kivonat -
József Kardos , Péter Závodszky , Yuji Goto , Gábor Náray-Szabó , László Gráf , Katalin Szilágyi , Orsolya Barabás , Anna Palló , Veronika Harmat , Péter Gál
Revisiting the mechanism of the autoactivation of the complement protease C1r in the C1 complex: Structure of the active catalytic region of C1r
Mol. Immunol. 45(6):1752-1760 (2008) Kivonat -
András L. Kiss , Balázs Hornung , Krisztina Rádi , Zsolt Gengeliczki , Bálint Sztáray , Tünde Juhász , Zoltán Szeltner , Veronika Harmat , László Polgár
The acylaminoacyl peptidase from Aeropyrum pernix K1 thought to be an exopeptidase displays endopeptidase activity
J. Mol. Biol. 368: 509-520. (2007) Kivonat -
László Beinrohr , Veronika Harmat , József Dobó , Zsolt Lörincz , Péter Gál , Péter Závodszky
C1 inhibitor serpin domain structure reveals the likely mechanism of heparin potentiation and conformational disease
J. Biol. Chem. 282: 21100-21109. (2007) Kivonat -
Krisztián Fodor , Veronika Harmat , Csaba Hetényi , József Kardos , József Antal , András Perczel , András Patthy , Gergely Katona , László Gráf
Extended intermolecular interactions in a serine protease canonical inhibitor complex account for strong and highly specific inhibition
J. Mol. Biol. 350:156-169. (2005) Kivonat -
István Horváth , Veronika Harmat , András Perczel , Villő Pálfi , László Nyitray , Attila Nagy , Emma Hlavanda , Gábor Náray-Szabó , Judit Ovádi
The structure of the complex of calmodulin with KAR-2: a novel mode of binding explains the unique pharmacology of the drug
J. Biol. Chem. 280(9):8266-8274. | PMID: 15596444 (2005) Kivonat -
Márton Megyeri , Veronika Harmat , Balázs Major , Ádám Végh , Júlia Balczer , Dávid Héja , Katalin Szilágyi , Dániel Datz , Gábor Pál , Péter Závodszky , Péter Gál , József Dobó
Quantitative characterization of the activation steps of Mannan-Binding Lectin (MBL)-Associated Serine Proteases (MASPs) points to the central role of MASP-1 in the initiation of the complement lectin pathway
J Biol Chem 288(13):8922-8934. | DOI: 10.1074/jbc.M112.446500 | PMID: 23386610 (2013) Kivonat -
Ibolya Leveles , Veronika Németh , Judit E. Szabó , Veronika Harmat , Kinga Nyíri , Ábris Ádám Bendes , Veronika Papp-Kádár , Imre Zagyva , Gergely Róna , Olivér Ozohanics , Károly Vékey , Judit Tóth , Beáta G. Vértessy
Structure and enzymatic mechanism of a moonlighting dUTPase
Acta Crystallogr D Biol Crystallogr. D69:2298-2308. | DOI: 10.1107/S0907444913021136 | PMID: 24311572 (2013) Kivonat -
Péter Rapali , László Radnai , Dániel Süveges , Veronika Harmat , Ferenc Tölgyesi , Weixiao Y. Wahlgren , Gábor Pál
Directed evolution reveals the binding motif preference of the LC8/DYNLL hub protein and predicts large numbers of novel binders in the human proteome
PLoS One 6(4):e18818. | DOI: 10.1371/journal.pone.0018818 | PMID: 21533121 (2011) Kivonat -
Ildikó Pécsi , Ibolya Leveles , Veronika Harmat , Beáta G. Vértessy , Judit Tóth
Aromatic stacking between nucleobase and enzyme promotes phosphate ester hydrolysis in dUTPase
Nucleic Acids Res. 38(20):7179-7186. | DOI: 10.1093/nar/gkq584 | PMID: 20601405 (2010) Kivonat -
Károly Kánai , Péter Arányi , Zsolt Böcskei , György Ferenczy , Veronika Harmat , Kálmán Simon , Sándor Bátori , Gábor Náray-Szabó , István Hermecz
Prolyl oligopeptidase inhibition by N-acyl-pro-pyrrolidine-type molecules
J Med Chem. 51(23):7514-7522. | DOI: 10.1021/jm800944x | PMID: 19006380 (2008) Kivonat -
Krisztián Fodor , Veronika Harmat , Richard Neutze , László Szilágyi , László Gráf , Gergely Katona
Enzyme:substrate hydrogen bond shortening during the acylation phase of serine protease catalysis
Biochemistry 45(7):2114-2121. | 10.1021/bi0517133 | PMID: 16475800 (2006) Kivonat -
Péter Gál , Andrea Kocsis , Tünde Bián , Veronika Harmat , László Barna , Géza Ambrus , Barbara Végh , Robert B. Sim , Péter Závodszky , Gábor Náray-Szabó
A true autoactivating enzyme. Structural insight into mannose-binding lectin-associated serine protease-2 activations
J. Biol. Chem. 280(39):33435-33444. | PMID: 16040602 (2005) Kivonat -
Veronika Harmat , Péter Gál , József Kardos , Katalin Szilágyi , Géza Ambrus , Gábor Náray-Szabó , Péter Závodszky
The structure of MBL-associated serine protease-2 reveals that identical substrate specificities of C1s and MASP-2 are realized through different sets of enzyme-substrate interactions
J. Mol. Biol. 342(5):1533-1546. | PMID: 15364579 (2004) Kivonat -
Veronika Harmat , Zsolt Böcskei , Gábor Náray-Szabó , Imre Bata , Andrea S. Csutor , István Hermecz , Péter Arányi , Beáta Szabó , Károly Liliom , Beáta G. Vértessy
A new potent calmodulin antagonist with arylalkylamine structure: crystallographic, spectroscopic and functional studies
J. Mol. Biol. 297(3):747-755. | DOI: 10.1006/jmbi.2000.3607 | PMID: 10731425 (2000) Kivonat -
Beáta G. Vértessy , Veronika Harmat , Gábor Náray-Szabó , Zsolt Böcskei , Ferenc Orosz , Judit Ovádi
Simultaneous binding of drugs with different chemical structures to Ca2+-calmodulin: crystallographic and spectroscopic studies
Biochemistry 37(44):15300-15310. | DOI: 10.1021/bi980795a | PMID: 9799490 (1998) Kivonat -
Dóra K. Menyhárd , Zoltán Orgován , Zoltán Szeltner , Ilona Szamosi , Veronika Harmat
Catalytically distinct states captured in a crystal lattice: the substrate-bound and scavenger states of acylaminoacyl peptidase and their implications for functionality
Acta Crystallogr D Biol Crystallogr. 71(Pt 3):461-472. | DOI: 10.1107/S1399004714026819 | PMID: 25760596 (2015) Kivonat -
Viktória Goldschmidt Gőz , István Pintér , Veronika Harmat , András Perczel
Approaches to Pyranuronic beta-Sugar Amino Acid Building Blocks of Peptidosaccharide Foldamers
Eur. J. Org. Chem. 355-361. DOI: 10.1002/ejoc.201701612 (2018) Kivonat -
Tibor Pasinszki , Dániel Dzsotján , Győző György Lajgut , Veronika Harmat , Antal Csámpai , Andrea Bor , István Zupkó
Synthesis, spectral- and theoretical study, x-ray analysis, and antiproliferative activity of 4,5-dihydrobenzoferroceno[1,2-d][1,2,3]selenadiazole and its benzo-fused analogue
Journal of Organometallic Chemistry 863:70-76. | DOI: 10.1016/j.jorganchem.2018.03.031 (2018) Kivonat -
Roland Szalay , Veronika Harmat , János Eőri , Gábor Pongor
Strong influence of intramolecular Si⋯O proximity on reactivity: Systematic molecular structure, solvolysis, and mechanistic study of cyclic N-trimethylsilyl carboxamide derivatives
Tetrahedron Letters 58(23):2186-2192. | DOI: 10.1016/j.tetlet.2017.04.057 (2017) Kivonat -
V. Bugris , Cs. Dudás , B. Kutus , V. Harmat , K. Csankó , S. Brockhauser , I. Pálinkó , Peter Turner , P. Sipos
Crystal and solution structures of calcium complexes relevant to problematic waste disposal: calcium gluconate and calcium isosaccharinate
Acta Crystallographica Section B 598-609 | doi.org/10.1107/S2052520618013720 (2018) Kivonat -
Anikó Angyal , András Demjén , Veronika Harmat , János Wölfling , László G. Puskás , Iván Kanizsai
1,3-Dipolar Cycloaddition of Isatin-Derived Azomethine Ylides with 2H‐Azirines: Stereoselective Synthesis of 1,3- Diazaspiro[bicyclo[3.1.0]hexane]oxindoles
The Journal of Organic Chemistry 4273-4281 DOI: 10.1021/acs.joc.9b00242 (2019) Kivonat -
Valéria Bugris , Veronika Harmat , Györgyi Ferenc , Sándor Brockhauser , Ian Carmichael , Elspeth F. Garman
Radiation-damage investigation of a DNA 16-mer
Journal of Synchrotron Radiation 998-1009 doi.org/10.1107/S160057751900763X (2019) Kivonat -
Zsolt Dürvanger , Veronika Harmat
Structural Diversity in Calmodulin - Peptide Interactions
Current Protein & Peptide Science 1102 - 1111 doi.org/10.2174/1389203720666190925101937 (2019) Kivonat -
Anna J. Kiss-Szemán , Veronika Harmat , Dóra K. Menyhárd
Achieving Functionality Through Modular Build-up: Structure and Size Selection of Serine Oligopeptidases
Current Protein & Peptide Science 1089 - 1101 DOI:10.2174/1389203720666190925103339 (2019) Kivonat -
Kinga Judit Fodor , Dániel Hutai , Tamás Jernei , Angéla Takács , Zsófia Szász , Máté Sulyok-Eiler , Veronika Harmat , Rita Oláh Szabó , Gitta Schlosser , Ferenc Hudecz , László Kőhidai , Antal Csámpai
Novel Polycondensed Partly Saturated β-Carbolines Including Ferrocene Derivatives: Synthesis, DFT-Supported Structural Analysis, Mechanism of Some Diastereoselective Transformations and a Preliminary Study of their In Vitro Antiproliferative Effects
Molecules 25(7), 1599 | doi: 10.3390/molecules25071599 (2020) Kivonat -
Anita Kapros , Attila Balázs , Veronika Harmat , Adrienn Háló , Lívia Budai , István Pintér , Dóra K. Menyhárd , András Perczel
Configuration‐Controlled Crystal and/or Gel Formation of Protected d‐Glucosamines Supported by Promiscuous Interaction Surfaces and a Conformationally Heterogeneous Solution State
Chemistry—A European Journal 26 | doi.org/10.1002/chem.202000882 (2020) Kivonat -
Anna J. Kiss-Szemán , Pál Stráner , Imre Jákli , Naoki Hosogi , Veronika Harmat , Dóra K. Menyhárd , András Perczel
Cryo-EM structure of acylpeptide hydrolase reveals substrate selection by multimerization and a multi-state serine-protease triad
Chemical Science DOI: 10.1039/d2sc02276a (2022) Kivonat -
Anna J. Kiss-Szemán , Luca Takács , Zoltán Orgován , Pál Stráner , Imre Jákli , Gitta Schlosser , Simonas Masiulis , Veronika Harmat , Dóra K. Menyhárd , András Perczel
A carbapenem antibiotic inhibiting a mammalian serine protease: structure of the acylaminoacyl peptidase–meropenem complex
Chemical Science 13, 14264 - 14276 I DOI: 10.1039/D2SC05520A (2022) Kivonat -
Zsolt Dürvanger , Eszter Boros , Zoltán Attila Nagy , Rózsa Hegedüs , Márton Megyeri , József Dobó , Péter Gál , Gitta Schlosser , Annamária F. Ángyán , Zoltán Gáspári , András Perczel , Veronika Harmat , Gábor Mező , Dóra K. Menyhárd , Gábor Pál
Directed Evolution-Driven Increase of Structural Plasticity Is a Prerequisite for Binding the Complement Lectin Pathway Blocking MASP-Inhibitor Peptides
ACS Chem. Biol. 17(4), 969–986 I DOI: 10.1021/acschembio.2c00114 (2022) Kivonat -
Zoltán Attila Nagy , Dávid Héja , Dániel Bencze , Bence Kiss , Eszter Boros , Dávid Szakács , Krisztián Fodor , Matthias Wilmanns , Andrea Kocsis , József Dobó , Péter Gál , Veronika Harmat , Gábor Pál
Synergy of protease-binding sites within the ecotin homodimer is crucial for inhibition of MASP enzymes and for blocking lectin pathway activation
J. Biol. Chem. 298(6) 101985 I DOI: 10.1016/j.jbc.2022.101985 (2022) Kivonat -
Kende Attila Béres , Zoltán Homonnay , Libor Kvitek , Zsolt Dürvanger , Martina Kubikova , Veronika Harmat , Fanni Szilágyi , Zsuzsanna Czégény , Péter Németh , Laura Bereczki , Vladimir M. Petruševski , Mátyás Pápai , Attila Farkas , László Kótai
Thermally Induced Solid-Phase Quasi-Intramolecular Redox Reactions of [Hexakis(urea-O)iron(III)] Permanganate: An Easy Reaction Route to Prepare Potential (Fe,Mn)Ox Catalysts for CO2 Hydrogenation
Inorg. Chem. 61(36), 14403–14418 I DOI: 10.1021/acs.inorgchem.2c02265 (2022) Kivonat -
Kende Attila Béres , Zoltán Homonnay , Berta Barta Holló , Maria Gracheva , Vladimir M. Petruševski , Attila Farkas , Zsolt Dürvanger , László Kótai
Synthesis, structure, and Mössbauer spectroscopic studies on the heat‑induced solid‑phase redox reactions of hexakis(urea‑O)iron(III) peroxodisulfate
Journal of Materials Research 38, 1102–1118 I DOI: 10.1557/s43578-022-00794-w (2022) Kivonat -
Zsolt Dürvanger , Tünde Juhász , Károly Liliom , Veronika Harmat
Structures of calmodulin–melittin complexes show multiple binding modes lacking classical anchoring interactions
J. Biol. Chem. 299(4): 104596 I DOI: 10.1016/j.jbc.2023.104596 (2023) Kivonat -
Dániel Horváth , Zsolt Dürvanger , Dóra K. Menyhárd , Máté Sulyok-Eiler , Fruzsina Bencs , Gergő Gyulai , Péter Horváth , Nóra Taricska , András Perczel
Polymorphic amyloid nanostructures of hormone peptides involved in glucose homeostasis display reversible amyloid formation
Nature Communications 14, 4621 I https://doi.org/10.1038/s41467-023-40294-x (2023) Kivonat -
Kende Attila Béres , Zoltán Homonnay , Laura Bereczki , Zsolt Dürvanger , Vladimir M. Petruševski , Attila Farkas , László Kótai
Crystal Nanoarchitectonics and Characterization of the Octahedral Iron(III)–Nitrate Complexes with Isomer Dimethylurea Ligands
Crystals 13(7), 1019 I DOI: https://doi.org/10.3390/cryst13071019 I ISSN 2073-4352 (2023) Kivonat -
Kende Attila Béres , Fanni Szilágyi , Zoltán Homonnay , Zsolt Dürvanger , Laura Bereczki , László Trif , Vladimir M. Petruševski , Attila Farkas , Niloofar Bayat , László Kótai
Structural, Spectroscopic, and Thermal Decomposition Features of [Carbonatotetraamminecobalt(III)] Iodide—Insight into the Simultaneous Solid-Phase Quasi-Intramolecular Redox Reactions
Inorganics 11(2):68. I DOI: https://doi.org/10.3390/inorganics11020068 (2023) Kivonat -
Ho-Jin Lee , Shi-Wei Liu , Máté Sulyok-Eiler , Veronika Harmat , Viktor Farkas , Zoltán Bánóczi , Mouna El Khabchi , Hua-Jun Shawn Fan , Kimihiko Hirao , Jong-Won Song
Neighbor effect on conformational spaces of alanine residue in azapeptides
Heliyon 10 (12), e33159 (2024) Kivonat -
Zsolt Dürvanger , Fruzsina Bencs , Dóra K. Menyhárd , Dániel Horváth , András Perczel
Solvent induced amyloid polymorphism and the uncovering of the elusive class 3 amyloid topology
Communications Biology 7: 968 (2024) Kivonat -
Enikő Meiszter , Tamás Gazdag , Péter J. Mayer , Attila Kunfi , Tamás Holczbauer , Máté Sulyok-Eiler , Gábor London
Revisiting Hafner’s Azapentalenes: The Chemistry of 1,3-Bis(dimethylamino)-2-azapentalene
J. Org. Chem. 89 (9), 5941-5951 | DOI: 10.1021/acs.joc.3c02564 (2024) Kivonat -
Máté Sulyok-Eiler , Veronika Harmat , András Perczel
Unravelling the Complexity of Amyloid Peptide Core Interfaces
J. Chem. Inf. Model. 64, 22, 8628–8640 I DOI: https://doi.org/10.1021/acs.jcim.4c01479 (2024) Kivonat -
Zoé S. Tóth , Ibolya Leveles , Kinga Nyíri , Gergely N. Nagy , Veronika Harmat , Thapakorn Jaroentomeechai , Oliver Ozohanics , Rebecca L. Miller , Marina Ballesteros Álvarez , Beáta G. Vértessy , András Benedek
The homodimerization domain of the Stl repressor is crucial for efficient inhibition of mycobacterial dUTPase
Sci Rep 14, 27171 I DOI: https://doi.org/10.1038/s41598-024-76349-2 (2024) Kivonat -
Szebasztián Szaniszló , Imre G. Csizmadia , Imre Jákli , Viktor Farkas , András Láng , Máté Sulyok-Eiler , Veronika Harmat , István Pintér , András Perczel
Intramolecular Inhibition by Imidazole in Acid-Catalyzed Hydrolysis of Protected Carbohydrates
Chem. Eur. J. 31(15), e202403319 (2025) Kivonat -
Kende Attila Béres , Zsolt Dürvanger , Zoltán Homonnay , Laura Bereczki , Berta Barta Holló , Attila Farkas , Vladimir M. Petruševski , László Kótai
Insight into the Structure and Redox Chemistry of [Carbonatotetraamminecobalt(III)] Permanganate and Its Monohydrate as Co-Mn-Oxide Catalyst Precursors of the Fischer-Tropsch Synthesis
Inorganics 12(4), 94 I DOI: https://doi.org/10.3390/inorganics12040094 (2024) Kivonat